Choose the reaction that represents the combustion of c6h12o2. – Combustion reactions, exemplified by the combustion of C6H12O2, play a pivotal role in various scientific and industrial processes. This article delves into the intricacies of this reaction, examining its chemical equation, energy changes, and practical applications, while highlighting factors that influence its efficiency and safety considerations.
Combustion Reaction of C6H12O2
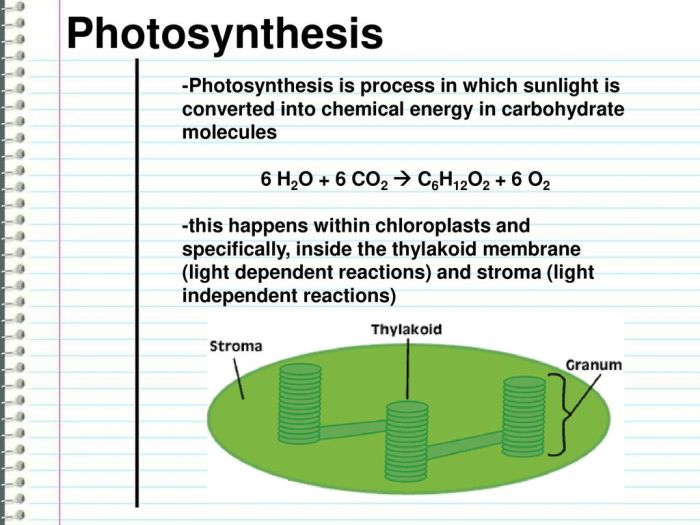
The combustion of C6H12O2, also known as hexyl acetate, is a chemical reaction that involves the burning of this organic compound in the presence of oxygen, releasing energy in the form of heat and light.
The chemical equation for the combustion of C6H12O2 is as follows:
C6H12O2 + 9O2 → 6CO2 + 6H2O + heat + light
In this reaction, C6H12O2 (hexyl acetate) is the fuel, while O2 (oxygen) is the oxidant. The products of the reaction are CO2 (carbon dioxide) and H2O (water), along with the release of heat and light energy.
The stoichiometry of the reaction indicates that one molecule of C6H12O2 reacts with nine molecules of O2 to produce six molecules of CO2 and six molecules of H2O. This stoichiometric ratio is important for ensuring complete combustion, where all of the fuel is consumed and converted into products.
Energy Changes in Combustion
The combustion of C6H12O2 is an exothermic reaction, which means that it releases energy in the form of heat and light. The energy released during combustion comes from the chemical bonds that are broken in the fuel molecules and formed in the product molecules.
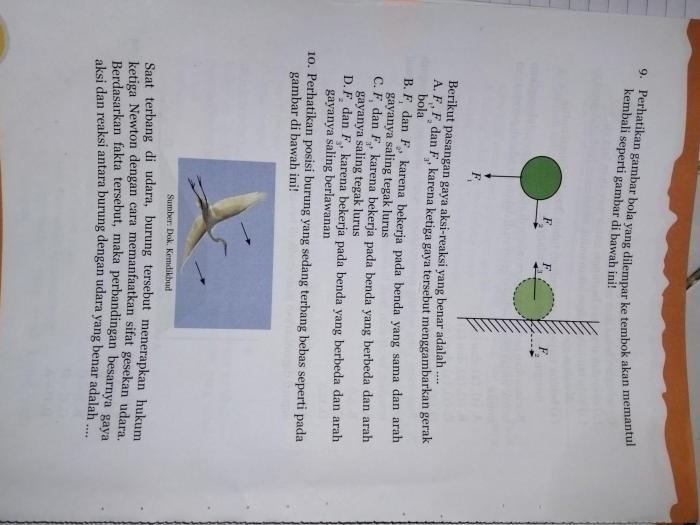
The heat released during combustion can be used to power engines, generate electricity, or provide warmth for homes and industries. The light released during combustion can be used for illumination or signaling purposes.
Factors Affecting Combustion

The rate and efficiency of the combustion of C6H12O2 can be affected by several factors, including:
- Temperature:Higher temperatures increase the rate of combustion, as they provide more energy for the reaction to occur.
- Pressure:Increased pressure can also increase the rate of combustion, as it forces the reactants closer together.
- Oxygen concentration:The availability of oxygen is essential for combustion to occur. Higher oxygen concentrations lead to faster and more efficient combustion.
- Catalysts:Catalysts are substances that can increase the rate of a reaction without being consumed themselves. In the case of C6H12O2 combustion, certain catalysts can help to break down the fuel molecules and promote faster reaction rates.
- Inhibitors:Inhibitors are substances that can slow down or prevent a reaction from occurring. In the case of C6H12O2 combustion, certain inhibitors can be used to reduce the rate of reaction and prevent uncontrolled combustion.
Applications of Combustion
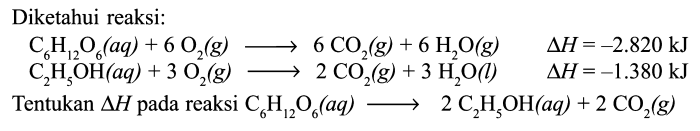
The combustion of C6H12O2 has a wide range of practical applications, including:
- Fuel source:C6H12O2 is used as a fuel in engines, power plants, and heating systems. It is a versatile fuel that can be used in both liquid and vapor form.
- Solvent:C6H12O2 is also used as a solvent for various purposes, such as in the production of paints, coatings, and adhesives.
- Flavoring agent:C6H12O2 is used as a flavoring agent in the food and beverage industry, imparting a fruity or floral aroma and taste.
Safety Considerations: Choose The Reaction That Represents The Combustion Of C6h12o2.
The combustion of C6H12O2 can pose certain safety hazards, including:
- Fire and explosion:C6H12O2 is a flammable liquid that can easily catch fire or explode if not handled properly.
- Toxic fumes:The combustion of C6H12O2 can produce toxic fumes, such as carbon monoxide and nitrogen oxides, which can be harmful to human health.
- Skin and eye irritation:C6H12O2 can cause skin and eye irritation upon contact.
To ensure safety when handling and combusting C6H12O2, proper precautions must be taken, such as:
- Proper storage:C6H12O2 should be stored in a cool, well-ventilated area away from sources of heat and ignition.
- Protective equipment:When handling C6H12O2, appropriate protective equipment, such as gloves, goggles, and a respirator, should be worn.
- Ventilation:Adequate ventilation is essential when combusting C6H12O2 to prevent the accumulation of toxic fumes.
- Emergency response plan:An emergency response plan should be in place to address potential hazards associated with C6H12O2 combustion.
FAQ
What is the chemical equation for the combustion of C6H12O2?
C6H12O2 + 6O2 → 6CO2 + 6H2O
How does temperature affect the combustion of C6H12O2?
Higher temperatures generally increase the rate of combustion, leading to more efficient and complete reactions.
What are the safety precautions that must be taken when handling C6H12O2?
C6H12O2 is a flammable liquid, so proper handling and storage are essential to prevent fires or explosions.