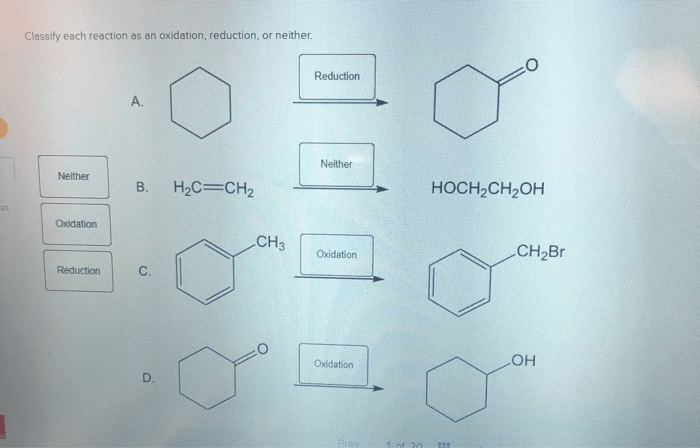
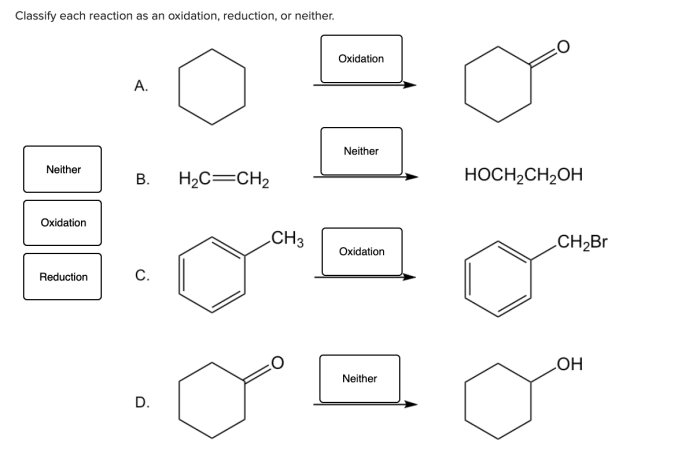
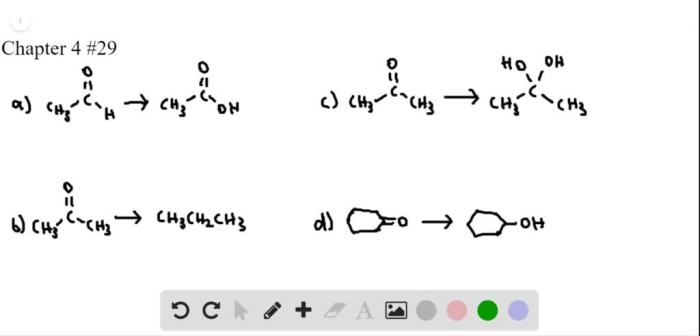
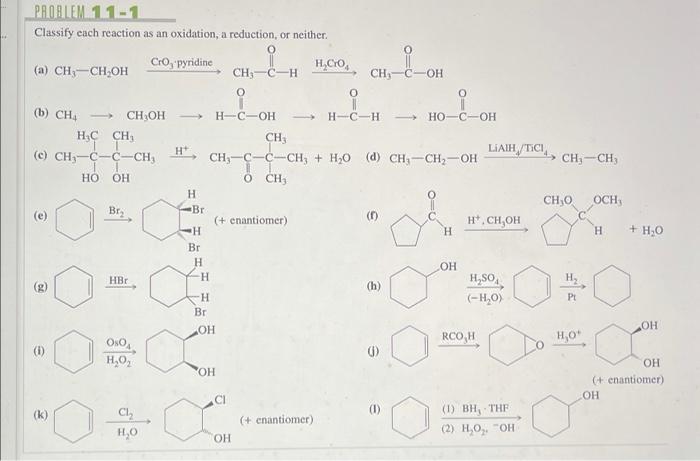
Classify each reaction as an oxidation reduction or neither – Delving into the realm of chemical reactions, we embark on an exploration of oxidation-reduction reactions, a fundamental concept that plays a pivotal role in myriad chemical processes. This discussion will delve into the intricacies of these reactions, providing a comprehensive understanding of their mechanisms and significance.
Chemical reactions, the cornerstone of chemistry, involve the transformation of reactants into products through the rearrangement of atoms and molecules. Among the diverse types of chemical reactions, oxidation-reduction reactions stand out as those involving the transfer of electrons between atoms or ions.
This intricate process, known as redox, underpins countless phenomena in both the natural world and industrial applications.
Chemical Reactions
Chemical reactions involve the transformation of substances into new substances. They occur when atoms or molecules interact, leading to the rearrangement of their constituent atoms.
Examples of chemical reactions include the combustion of fuels, the rusting of iron, and the photosynthesis in plants.
Types of Chemical Reactions, Classify each reaction as an oxidation reduction or neither
- Combination reactions:Two or more substances combine to form a single product.
- Decomposition reactions:A single substance breaks down into two or more products.
- Single-replacement reactions:An element replaces another element in a compound.
- Double-replacement reactions:Two compounds exchange ions to form two new compounds.
Oxidation-Reduction Reactions
Oxidation-reduction reactions involve the transfer of electrons between atoms or ions. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons.
Examples of oxidation-reduction reactions include the combustion of fuels, the rusting of iron, and the electrolysis of water.
Process of Oxidation and Reduction
In oxidation, an atom or ion loses electrons, resulting in an increase in its oxidation state. In reduction, an atom or ion gains electrons, resulting in a decrease in its oxidation state.
Classifying Reactions: Classify Each Reaction As An Oxidation Reduction Or Neither
| Reaction | Type | Oxidation | Reduction |
|---|---|---|---|
| 2H2 + O2 → 2H2O | Combination | H: 0 → +1 | O: 0 →
|
| CaCO3 → CaO + CO2 | Decomposition | C: +4 → +2 | O:
|
| Fe + 2HCl → FeCl2 + H2 | Single-replacement | Fe: 0 → +2 | H: +1 → 0 |
| AgNO3 + NaCl → AgCl + NaNO3 | Double-replacement | Ag: +1 → 0 | Cl: 0 →
|
Reactions are classified as oxidation-reduction if there is a change in the oxidation states of the atoms involved.
Applications of Oxidation-Reduction Reactions
Oxidation-reduction reactions play a vital role in everyday life and various fields:
- Energy production:Combustion of fuels, such as gasoline and natural gas, releases energy through oxidation-reduction reactions.
- Metallurgy:Extraction and purification of metals from their ores involve oxidation-reduction processes.
- Biological processes:Cellular respiration, photosynthesis, and many other biochemical reactions are based on oxidation-reduction.
- Electrochemistry:Batteries and fuel cells rely on oxidation-reduction reactions to generate electricity.
FAQ Compilation
What are the key criteria for classifying a reaction as oxidation or reduction?
The primary criterion is the change in oxidation number of the atoms involved. Oxidation involves an increase in oxidation number, while reduction involves a decrease.
How can we identify oxidation and reduction half-reactions in a redox reaction?
Oxidation half-reactions involve the loss of electrons, while reduction half-reactions involve the gain of electrons.
What is the significance of redox reactions in everyday life?
Redox reactions are essential for processes such as respiration, photosynthesis, and the functioning of batteries.